“It's getting to the point of wondering what GLP-1 agonists aren't good for.”
What started as a diabetes & weight loss drug is snowballing with momentum as it becomes increasingly relevant for multiple other age-related diseases. Or rather excitingly, a glimmer of what an aging drug to the masses can look like.
Research around reversing or curing aging is a radically different quest from commercializing known biology to restore a specific functional decline or prevent onset of age-related diseases.
Drugs promoting metabolic fitness (GLP-1s and next-gen tx) have the potential to become the first multi-morbidity, preventative drugs. Even more interesting: promoting metabolic fitness = compelling science for lifespan extension
I wrote the below review on metabolic mechs & aging in 2021 w/ a few amendments revisiting it recently.
Metabolic Mechanisms and Aging
Metabolism = set of chemical processes that convert food ⇒ energy
In simplest terms, metabolic mechanisms keep the engine of your body “running”. Over decades of research, we’ve observed that twisting this knob (i.e. caloric restriction, IGF-1 inhibition) can manipulate lifespan in model organisms (worms, mice, dogs).
Metabolic fitness = how well the body generates, uses, and stores energy
Metabolic fitness declines with age, creating dysfunction in the chain of conversion from food to energy.
The flow:
Eat food ⇒ Nutrient sensing/uptake ⇒ Convert to energy ⇒ Use energy
A dysfunctional flow is tied to acute diseases and lifespan. Diseases of metabolic decline:
Obesity (dysfunctional fat storage)
Insulin resistance (dysfunctional glucose uptake)
Cardiovascular diseases like heart attack and stroke (cardiac inflammation and insulin resistance)
Alzheimer’s disease (loss of proteostasis & insulin resistance in the brain, in addition to other factors known & unknown)
Nutrient Sensing Pathways and Aging
Your cells generate energy from the food you eat, but the carbohydrates, proteins, and fats are first broken down into their smaller component units (monomers).
carbohydrates ⇒ sugars (i.e. glucose)
proteins ⇒ amino acids
fats ⇒ fatty acids
Your body detects and regulates the levels of each nutrient. An increase in [monomers] will trigger your cells to take up nutrients. Each macromolecule (i.e. glucose) has a unique set of pathways that monitor and regulate its levels in the body. Many of these core nutrient sensing pathways are involved in the regulation of lifespan.
Insulin and GH/IGF-1 signaling
Insulin is involved in several signaling pathways that are control points for aging—one of the most prominent is the growth hormone/insulin-like growth factor 1 (GH/IGF-1) signaling pathway.
Suppressing GH/IGF-1 hormone levels extends lifespan. Both GH and IGF-1 deficient mice have significantly longer lifespans than regular mice.
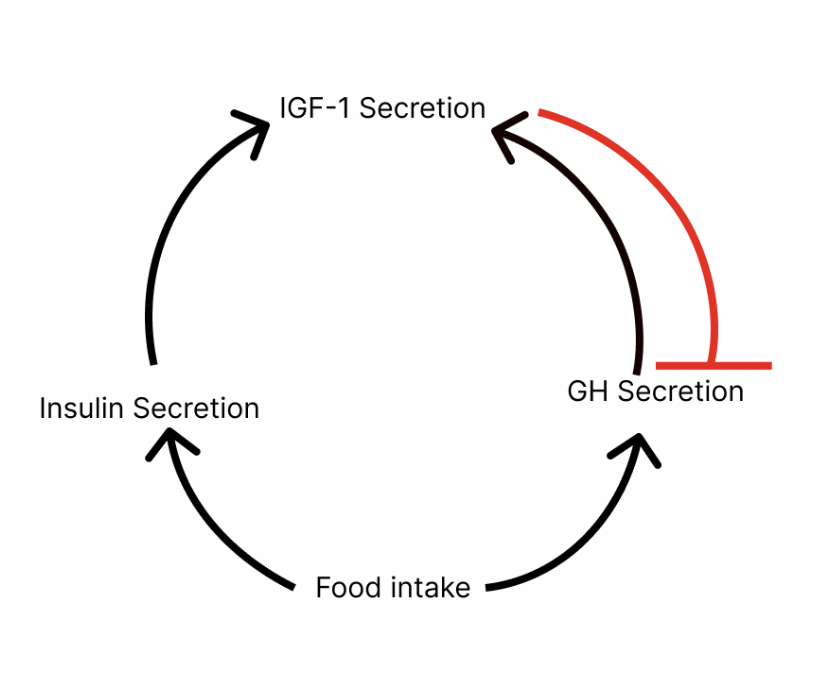
GH/IGF-1 signaling stimulates systemic body growth. Both hormones are regulated by a feedback loop:
Food intake triggers GH and insulin secretion which triggers secretion of IGF-1 (hormone that stimulates tissue and bone growth).
Increasing levels of IGF-1 inhibit GH secretion (to prevent further IGF-1 release).
GH levels change throughout your life: they’re highest during the pubertal/early adulthood period and decline with age. Rather confusingly, age-associated decline in GH/IGF-1 signaling is linked with an onset of aging symptoms (i.e. loss of muscle mass, reduced bone mineral density, decline in energy levels). If you thought biology was gonna be straightforward, tough luck.
Suppressing GH/IGF-1 signaling increases lifespan. But suppression of IGF-1 at a specific point in life is key. IGF-1 suppression at a young age is beneficial for longer lifespan. This is why caloric restriction, which reduces both insulin and IGF-1 levels, is most beneficial when started in early to mid-life.
Drugs that can mimic the effects of caloric restriction (& suppress the insulin/IGF-1 pathway) like Metformin can extend lifespan.
AMPK Signaling
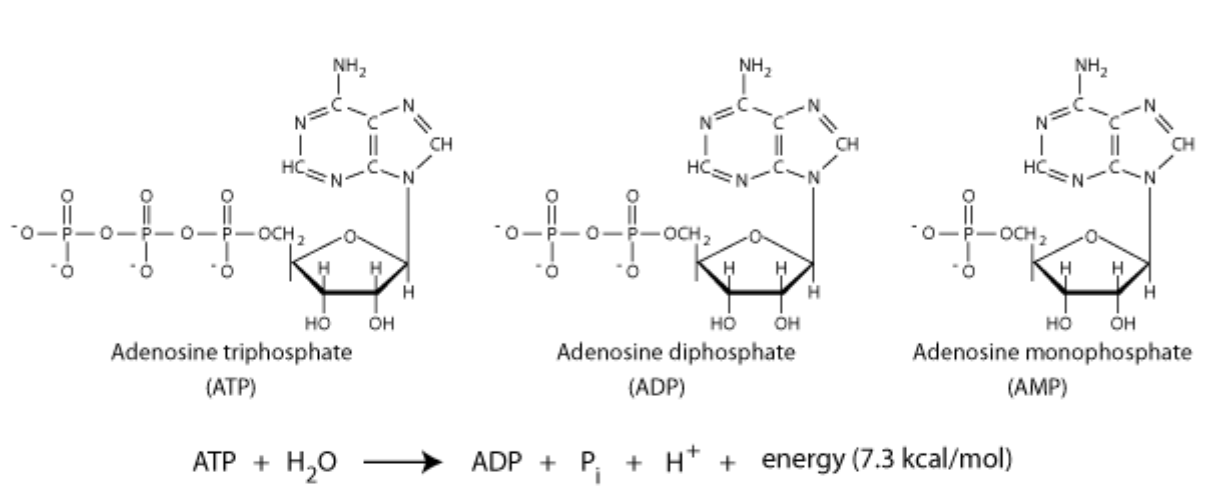
Increased AMPK activity = longer lifespan. Adenosine monophosphate-activated protein kinase (AMPK) helps regulate cell energy levels. Its mechanisms are activated by a decrease in cell energy levels.
Cells store energy in the form of ATP: during cellular respiration, the molecule adenosine monophosphate (AMP) is converted to adenosine triphosphate (ATP). Energy is later released by breaking down ATP via hydrolysis.
AMPK tracks the cell ratio of AMP/ATP to monitor available energy levels in the body. If you’re nutrient-deprived, you have higher AMP / lower ATP. This activates AMPK which helps generate more ATP by decreasing energy expenditure, suppressing protein synthesis, and promoting cell autophagy.
AMPK agonists such as Metformin and Berberine are well-known drugs researched for lifespan extension.
mTOR
Mammalian/mechanistic target of rapamycin (mTOR) is a protein that regulates cell growth, autophagy, and protein synthesis. mTOR is composed of two complexes: mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2).
mTORC1 acts as a nutrient sensor involved in regulating both, glucose and amino acid homeostasis:
mTORC1 activity is receptive to glucose sensing. Insulin/IGF-1 signaling (IIS) activates several pathways that converge and activate mTORC1.
mTORC1 acts as a sensor for amino acid levels. mTORC1 activation is partially regulated by amino acids— increasing amino acid levels increasingly activate the complex which promotes protein synthesis from the amino acids.
Inhibiting mTORC1 activity extends lifespan. This pathway can be observed indirectly (i.e. diet restriction suppresses the IIS/mTOR pathway and promotes longer lifespan). Or directly (i.e. Rapamycin, another well-known longevity drug, extends lifespan by inhibiting mTORC1).
Nutrient-Regulated Adipokines
Adipokines, hormones secreted by adipocytes, are involved in systemic regulation of appetite, energy expenditure, and maintaining nutrient homeostasis. Dysregulation of these adipokines leads to cardiovascular disease, insulin resistance, sarcopenia, and other symptoms of biological aging. Altering adipokine levels is another potential strategy that can affect lifespan.
Leptin is an adipokine that inhibits appetite-stimulating neuropeptides and neurotransmitters. Leptin levels are positively correlated with lipid levels in the body— they typically increase with fat and obesity. In contrast, adiponectin is inversely correlated with lipid levels in the body. High levels of adiponectin are associated with insulin sensitivity and increased energy use, both markers of longer lifespan. Overexpression and augmentation of adiponectin is linked to longevity while adiponectin-deficiency is associated with shorter lifespan.
What happens to the nutrients once detected in the body?
Nutrient-sensing systems are responsible for alerting the body to presence + level of nutrients. Once the macromolecules/nutrients are detected, they’re either converted to energy or stored for future use in various organs. Dysfunction in these processes can also contribute to shorter lifespans and associated aging phenotypes.
Dysfunction with Glucose Uptake (manifests as insulin resistance, diabetes)
After you eat, the increase in blood glucose levels stimulates the pancreas to release insulin which signals glucose uptake by your cells. Ideally, this system maintains constant blood glucose levels in the body, but chronically high levels of insulin can desensitize cells to insulin effects. Cells absorb less glucose, instead leaving it in the blood. The high glucose levels trigger more insulin release leading to high levels of both, insulin and glucose, in the blood. This phenomenon is called insulin resistance which leads to further age-associated diseases such as cancer, cardiovascular diseases, and stroke.
Frequency of insulin resistance increases with age. High levels of insulin also mean increased insulin/IGF-1 signaling and exacerbation of aging phenotypes. Increased insulin sensitivity (and consequently, lower levels of circulating insulin) extends lifespan and has been suggested to be a marker of longevity.
Dysfunction with Fat/Lipid Storage (manifests as obesity, more visceral fat)
Adipose tissue is the largest organ involved in energy storage. It’s responsible for storing lipids, triglycerides, and other forms of fat that are broken down during periods of fasting, starvation, exercise, or other heightened energy demands. Adipose tissue changes with age in mass, cellular composition, and secretory profiles. Age-related dysfunction of adipose tissue plays a key role in development of inflammation and insulin resistance ⇒ aging phenotypes. Dysfunction in fat storage and adipokine secretion lead to metabolic diseases and shortened lifespan.
Changes in Adipose Secretory Profiles
Adipose tissue dysfunction leads to dysregulation of adipokines such as leptin and adiponectin, as discussed above. A decline in adiponectin levels correlates with the trend of increased obesity and insulin resistance.
Changes in Adipose Tissue Mass Distribution
Adipose tissue is distributed into different regions in the body: subcutaneous fat is under the skin and visceral fat is near internal organs. Adipose tissue distribution also changes with age, and the change in distribution contributes to increased risk of insulin resistance and shorter lifespans.
Visceral fat tissue mass is associated with insulin resistance, cardiovascular diseases, and all-cause mortality. Subcutaneous fat tissue has no correlation with disease risk. Typically, as you get older, distribution of adipose shifts more heavily to visceral fat.
Loss of Proteostasis
Amino acids are used to create proteins in a complex process. Protein folding is an integral aspect of protein synthesis. Misfolded proteins aren’t functional and are typically re-folded or degraded by chaperones (enzymes that help regulate proteostasis). Without degradation, misfolded proteins accumulate and clump together which is characteristic of neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Dysfunction of protein homeostasis (proteostasis) leads to more accumulation of protein aggregates. Proteostasis typically declines with age through increased misfolding and protein aggregation. Why?
As you age, the rate of protein synthesis decreases while oxidative damage to existing proteins increases. Molecular chaperones (which assist protein folding) become increasingly busy with fixing damaged proteins, rather than facilitating new protein folding. This leads to more frequent misfolding of new proteins to create damaged and poorly functional proteins.
Conversion to usable energy (Mitochondrial Dysfunction)
Nutrient intake is typically converted into energy within the mitochondria. Mitochondria produce energy in the form of ATP, and reactive oxygen species (ROS) accumulate as a side product. ROS are molecules known to induce oxidative damage in the body, and as a producer of ROS, mitochondria are prone to oxidative damage simply due to proximity. Oxidative damage can result in mitochondrial dysfunction.
Mitochondria regulate apoptosis (cell death) and autophagy (degradation of cell organelles) which maintains homeostasis within a cell and among cells. Dysfunction of this regulation leads to diseases such as cancer and sarcopenia. Mitochondrial dysfunction has also been linked to neurodegenerative diseases (neurons have high energy demand, making them sensitive against mitochondrial dysfunction). Mitochondrial dysfunction is characteristic of Alzheimer’s, Parkinson’s, and Huntington’s Disease.
Energy Expenditure and Aging
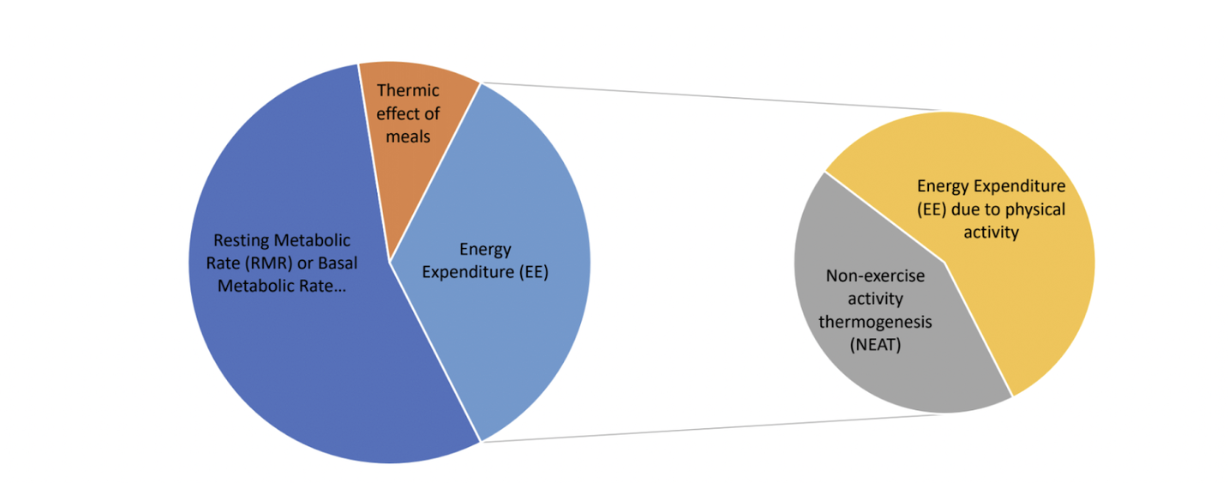
Once the food has been converted into usable energy, the body uses it in 4 ways:
Basal metabolic rate*
Even when you’re completely at rest, you use energy to keep the body functioning (i.e. breathing, cell growth, hormone release). The amount of energy you expend to ‘keep your body running’ is your basal metabolic rate. This rate varies by individual based on factors such as age, body size and composition, and sex.
*Also known as resting metabolic rate
Energy expenditure for physical activity
This is energy used for running, dancing, and any other sport-like physical exercise.
Energy expenditure for non-exercise activity thermogenesis (NEAT)
NEAT accounts for all energy spent for things we do that are not sleeping, eating, or sports-like exercise. Examples include typing, shivering, standing, or sitting.
Thermic effect of meals
The process of converting food into energy requires energy itself (as you’ve just read how complex the processes that go into it!) You spend energy to make more energy.
Total energy expenditure comprises all of these components. The disruptions in the metabolic process that we’ve discussed impede the body’s ability to store energy.
In addition to dysfunction during conversion of energy, the expenditure of energy is a control knob for lifespan. The rate that your body uses energy is metabolic rate, and this affects lifespan.
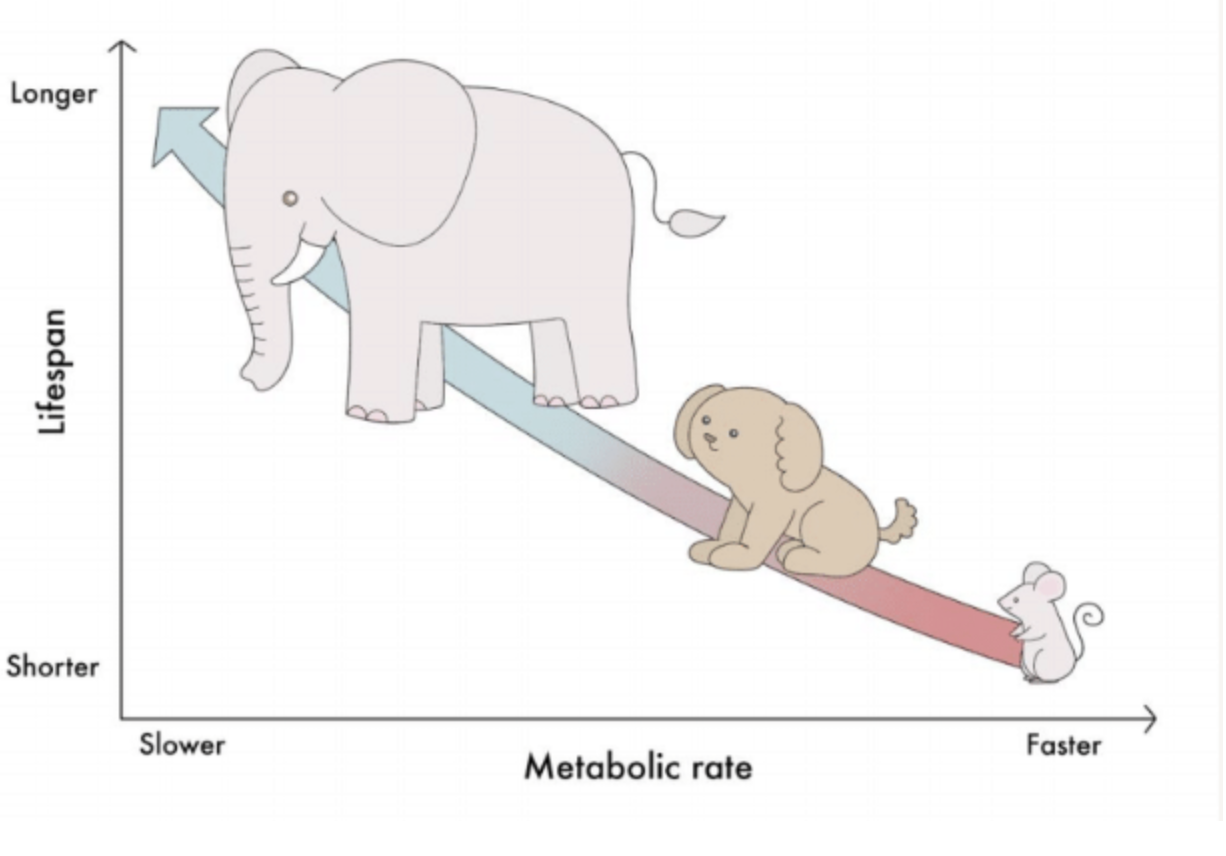
Lower basal metabolic rate = longer lifespan.
In 1908, scientist Max Rubner proposed that basal metabolic rate was proportional to body mass, and total energy expenditure, as a function of body size, was fixed. Finite energy used up quickly leads to a shorter lifespan. This led to the Rate of Living theory: higher rate of basal metabolism = shorter lifespan.
This trend is generally* supported by organisms. Smaller animals have higher BMR/unit mass than larger animals due to heat loss from a greater surface area:volume ratio. When looking at metabolic rate per unit of body mass, larger animals such as elephants have lower metabolic rates and longer lifespans than smaller animals such as mice.
*Note: There are some exceptions to this trend: some species of birds and bats are long-lived despite their high basal metabolic rate. This might be due to several reasons. 1) Shorter lifespans in the wild may not be due to biological aging. They could easily be killed off by predators or the environment. 2) Basal metabolic rate doesn’t encompass total energy metabolism. External factors, such as habitat, can skew energy expenditure beyond theoretical energy use per body size. For example, animals in colder environments tend to live longer. Independent of body size and BMR, animals in colder habitats will expend more total energy per gram of tissue.
Higher activity-based energy expenditure (physical exercise and NEAT) = longer lifespan
Studies have shown that increased activity energy expenditure is associated with longer lifespans. This seems intuitive to what most of us know: exercise is healthy for you. However, increased non-exercise based activity energy is also tied to longer lifespans. More energy spent shivering, standing, (yes, even typing) contribute to longevity.
UCP1 is a gene responsible for non-shivering thermogenesis (heat production) in cold weather and is restricted to expression in brown fat mitochondria. Studies showed that uncoupling expression of UCP1 from brown fat, which removes the restriction on expression, leads to significant increase in lifespan.
Higher total energy expenditure = longer lifespan
Your total energy expenditure increases leading up to adolescence and typically declines with age. Studies have shown that increased total energy expenditure is associated with longevity. Thus, age-associated decline in total energy expenditure correlates to mortality.
So, from a metabolic standpoint, living longer means…
Increase total energy expenditure. And adjust the proportions of energy usage to:
Increase activity and non-activity based energy expenditure
Decrease basal metabolic rate